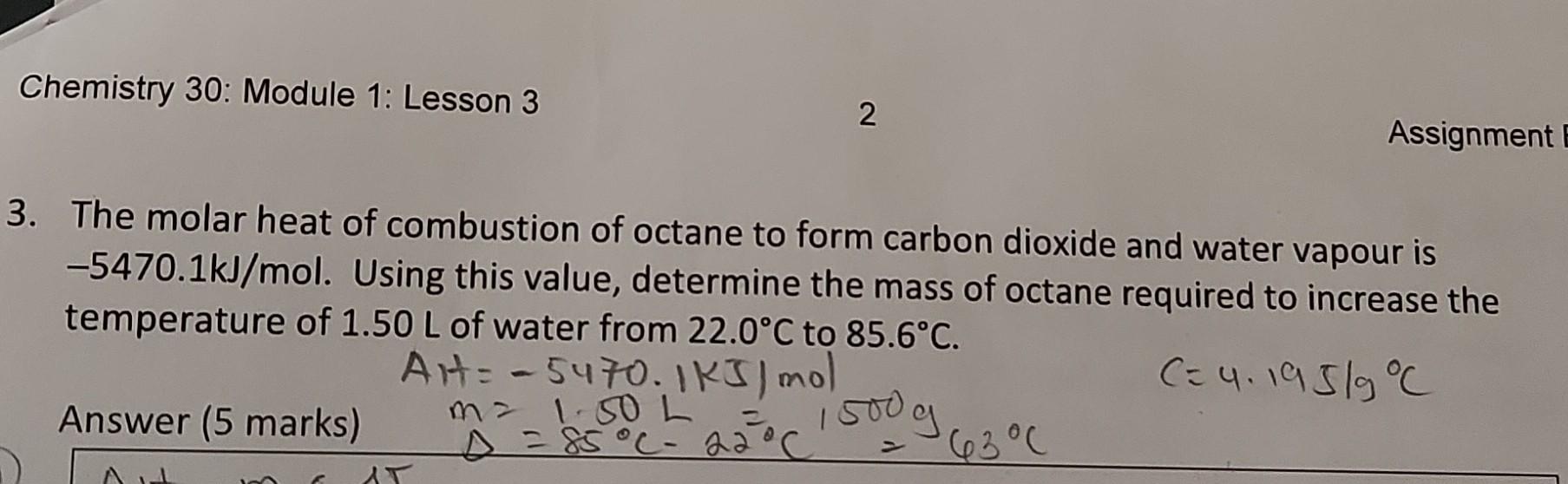RI ) whicho correct. 1 RT (NEET-I 2016) The heat of combustion of carbon to co, e_393.5 kJ/mol. The heat released upon formation of 35.2 g of CO2 from carbon and oxygen
37. the heat of combustion of C,S carbondisulphide 393.3, 293.7 1108.78KJ.What will be heat of formation of carbondisulphide

Enthalpy of combustion of alcohols data trend graph explaining trend pattern determining delta H combustion comparison with ethers equations advanced A level organic chemistry revision notes doc brown

The heat of the combustion of graphite and carbon monoxide respectively are 393.5 kJ mol1 and 283 kJ mol −1. Thus heat of formation of carbon monoxide in kJ mol −1 is:
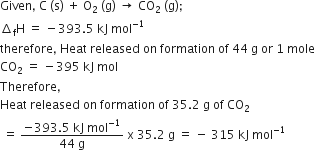
The heat of combustion of carbon to CO2 is -395.5 kJ/mol. The heat released upon the formation of 35.2 g CO2 from carbon and oxygen gas is - Zigya

74) If the heat of combustion of carbon monoxide constant volume and 17°C is -283.3 kJ, then its heat of combustion constant pressure (R = 8.314 J degree-1 mol-)) a) -284.5 kJ

enthalpy of combustion of carbon to co2 is -393 5 kilo joule per mole calculate the heat produced upon the - Chemistry - Thermodynamics - 13291531 | Meritnation.com

Enthalpy of combustion of carbon to CO2 is -393.5KJ mol-1. Calculate the heat released upon..... - YouTube
The combustion enthalpies of carbon, hydrogen, and methane are 395.5 kJ mol^ 1, 284.8 kJ mol^ 1 and 890.4 kJ mol^ 1 respectively at 25^0C. The value of s†an dard formation enthalpies
43. Calculate standard heat of formation of CS2. Given that standard heat of combustion of C, S and CS2 are –393.3, –293.72 and –1108.76 kJ mol–1
The combustion enthalpie of carbon hydrogen and methane are -395.5 kJ mol^-1 -284.8 kJ mol^-1 and -890.4 kJ - Sarthaks eConnect | Largest Online Education Community

1. The heats of combustion of carbon, hydrogen and acetylene are -394K.J, - 286K.J and -1301 K.J respectively. Calculate heat of formation of CH2 1) 621 KJ 3) -227 KJ 2) 454 KJ 4) 227 K.J